ABSTRACT
The plant tissue culture technology paved the way for in vitro callus cultures. Callus is an irregular, unorganised and undifferentiated mass of actively dividing cells. In vitro callus cultures can be exploited for the production of important metabolites from various plant organs like roots, leaves, flowers similar to the plants from the natural habitat. This will help in minimizing the uprooting of plants, maintaining the biodiversity and help in the conservation of important plat species. The quality and quantity of callus dependents on many factors like nature of the explants, media, nutritional requirements, plant growth regulators, carbon source, culture condition etc. In the present investigation, we have selected Spilanthes acmella Murr. commonly known as toothache plant, an important medicinal plant belonging to family Asteraceae. It is widely distributed in tropical and subtropical regions of the world. It has been reported to possess various biological activities like antipyretic, antidiuretic, anti-inflammatory, antioxidant, and immunomodulatory, hepatoprotective, anticancer and antitoothache. The plant has been found to produce important secondary metabolites like spilanthol, scopoletin, myrecene, α amyrin, β amyrin etc. Hence, the present study has been under taken to standardize the various parameters required for callus induction of S.acmella and its growth. The protocol for callus induction has been standardized which can serve as an important tool for production of important secondary metabolites of this medicinal plant. Also the callus cultures can be used for in vitro plant production which can help the plant to combat the threat of over exploitation and extinction.
Keywords: Spilanthes acmella; Callus Induction; Plant Tissue Culture; Medicinal Plants; Leaf and Nodal Explants
INTRODUCTION
Callus is an unorganised proliferative mass of cells produced from plant cells, tissues or organs when grown aseptically on artificial nutrient medium under controlled experimental conditions. Callus consists of loosely arranged parenchymatous cells arising from the proliferating cells of the cultured plants. The callus has a potential to develop into normal shoots, roots and embryoids. The nature and structure of the callus determines the morphogenic potential of the explants and serves as a basis for in vitro plant regeneration studied [1].
Spilanthes acmella Murr. is an important medicinal plant, found in tropical and subtropical countries mainly India and South America. Popularly, it is known as toothache plant which reduces the pain associated with toothaches and induces saliva secretion.
Different parts of this plant possess multiple pharmacological activities, which include antimicrobial, antipyretic, local anaesthetic, bio-insecticide, anticonvulsant, antioxidant, aphrodisiac, analgesic, diuretic, toothache relieve and anti-inflammatory effects [2]. Spilanthes acmella is an annual, erect or ascending stout herb, 20-50 cm high. The leaves are petiolate, arranged oppositely, broadly ovate, narrowed at base, acute at apex. The flowers in axillary or terminal, in head inflorescence, with involucre of bracts. The seeds are minute and black in colour. Flowering is generally from March to April.
S.acmella is a well-known anti-toothache plant and has been used as a traditional medicine for many purposes. The different plant parts of S.acmella like flowers heads, leaves, roots, stem and other aerial parts have been used in various health care systems [3]. Traditionally, Spilanthes plants are used to treat stammering in children, fungal skin diseases and remedy for snakebite.
S.acmella has been reported as acutely threatened and highly endangered plant species [4-5]. The plant is being over exploited for its medicinally important secondary metabolites. Hence there is a need to standardize callus culture protocols of this plant to serve as an alternate source of medicinally important secondary metabolites. Also the callus cultures can be used for in vitro plant production which can help the plant to combat the threat of over exploitation and extinction.
METHODOLOGY
Collection of Explants
For induction of callus, the nodal and leaf explants (young, mature and old) were collected from the field grown plants of S. acmella. The explants were surface sterilized and inoculated onto media with different concentrations of phytohormones.
Surface sterilization of explants
The various explants were collected from field grown plants of S.acmella like young, mature and old leaves, nodal segments, apical shoot tips and sterilized to remove the surface borne microorganisms. The explants were washed thoroughly under running tap water, treated with 1% bavistin for 20 minutes, followed by three rinses with distilled water. The explants were treated with Tween 20 (detergent) for 10 minutes and the material was repeatedly washed with sterile water. Then the explants were rinsed with 70% alcohol for 1 minute followed by distilled water washing twice.
The explants were then sterilized with 0.1 % mercuric chloride (HgCl 2 ) for 3 minutes under aseptic conditions followed by 3-4 washes with sterilized water to remove the traces of mercuric chloride. The explants were transferred aseptically onto different media and incubated in culture room with 25 ± 2ºC temperature and 16 hours photoperiod. For each experiment 3-4 replicates were maintained.
Callus Induction Studies
Surface sterilized explants i.e. leaves and nodal segments (young, mature and old) were transferred to a pre-sterilized petriplate then the material is cut in to pieces of appropriate size using sterilized scalpel. The cut sections were then placed on a sterilized filter paper to remove moisture.
Callus was induced from different explants of S.acmella i.e. leaves (young, mature and old) and nodal segments (young, mature and old). All the explants were inoculated onto MS media with various concentrations of 2,4-D (0.5-4 mg/l) alone and in combination with BAP (0.5 mg/l) and NAA(0.5-3.0 mg/l).
Three other different media namely Gamborgs’s Media (B5), MS with B5 vitamins, SH media were also tested to assess the role of media in callus induction. Callus, devoid of original explants were sub cultured at regular intervals of three weeks on to callus induction medium.
To determine the callus growth, the fresh weight and dry weight of callus was measured. The fresh weight of the callus was noted after four weeks of culture and dry weight of callus was taken after drying the callus in an oven at 750°C for 48 hours.
Standardization of media for callus induction
Culture media plays an important role in in vitro growth and morphogenesis of plant tissues. To standardize the media for callus induction and development, the leaf explants of S.acmella were inoculated onto four different media viz. MS, MS with B5 vitamins, SH, Gamborg’s media with 2.0 mg/l 2,4-D.
RESULTS
Callus induction from different explants of S.acmella
Different explants were inoculated onto MS Medium containing different concentrations of 2,4-D (0.5-4.0 mg/l) alone. The quality of callus was scored after 4 weeks of culture as low (+), moderate (++) and high (+++) based on the nature and growth of the callus. The frequency of calluing was calculated by counting the number of explants responded by total number of explants inoculated.
In leaf segments, callus was induced from all three types of explants i.e young, mature, old leaves (Table 1). Induction of callus with varying frequencies was observed from young, mature and old leaves on different concentrations of 2,4-D alone (Plate 1).
In young leaves, the percentage of callus ranged from 60 to 90 % among the different concentrations of 2,4-D alone.
Higher percentage of callus induction was observed at 2.5 mg/l of 2,4-D (90%). The callus produced was of moderate quality for lower concentrations of 2,4-D ( 0.5 mg/l and 1.0 mg/l) and also at 3.0 mg/l and 3.5 mg/l 2,4-D. High quality callus was produced at the concentrations of 1.5 mg/l, 2.0 mg/l and 2.5 mg/l 2,4-D. The induction and quality of callus was low at 4.0 mg/l concentration of 2,4-D.
For the mature leaves, the percentage of callusing varied from 60 to 80 % with different concentrations of 2,4-D. The maximum frequency of callusing (80%) was observed at 2.5 mg/l of 2,4-D.
The quality of callus was moderate at 0.5 mg/l to 1.5 mg/l 2,4-D and 3.0 to 3.5 mg/l 2,4-D. High percentage of callusing with good quality callus was observed at 2.0 mg/l and 2.5 mg/l 2,4-D. At 4.0 mg/l concentration of 2,4-D, low quality and low percentage of callus was observed.
For the old leaves, the callusing frequency varied from 30 to 50 % and maximum callusing percentage was noticed at 2.5 mg/l concentration of 2,4-D. The callus produced was of moderate quality at 0.5 mg/l to 2.5 mg/l 2, 4-D. Low quality callus was produced for concentrations 0.5, 1.0, 3.0 and 3.5 mg/l 2,4-D. The 2,4-D at 4.0 mg/l produced negligible amount of callus (20%).
Among all the explants tested, young leaves showed high callusing frequency (90 %) followed by Mature (80%) and Old leaves (50%). In young leaves, callus was induced within 7 days of inoculation. The mature and old leaves showed callus induction within 10 and 14 days of inoculation respectively.
For all the explants, the 2.5 mg/l concentration of 2,4-D induced highest percentage of callus induction.
|
S.No. |
Explants |
Hormone 2,4-D mg/l |
No. Of explants inoculated |
No. Of explants responded |
Callus induction % |
Quality of Callus |
|
1. |
Young Leaves |
0.5 |
10 |
6 |
60±0.32 |
++ |
|
1.0 |
10 |
7 |
70±0.21 |
++ |
||
|
1.5 |
10 |
8 |
80±0.45 |
+++ |
||
|
2.0 |
10 |
8 |
80±0.43 |
+++ |
||
|
2.5 |
10 |
9 |
90±0.25 |
+++ |
||
|
3.0 |
10 |
8 |
80±0.56 |
++ |
||
|
3.5 |
10 |
7 |
70±0.72 |
++ |
||
|
4.0 |
10 |
3 |
30±0.23 |
+ |
||
|
2. |
Mature Leaves |
0.5 |
10 |
6 |
60±0.62 |
++ |
|
1.0 |
10 |
5 |
50±0.44 |
++ |
||
|
1.5 |
10 |
6 |
60±0.43 |
++ |
||
|
2.0 |
10 |
7 |
70±0.67 |
+++ |
||
|
2.5 |
10 |
8 |
80±0.62 |
+++ |
||
|
3.0 |
10 |
7 |
70±0.24 |
++ |
||
|
3.5 |
10 |
5 |
50±0.38 |
++ |
||
|
4.0 |
10 |
2 |
20±0.21 |
+ |
||
|
3. |
Old Leaves |
0.5 |
10 |
3 |
30±0.29 |
+ |
|
1.0 |
10 |
3 |
30±0.32 |
+ |
||
|
1.5 |
10 |
3 |
30±0.46 |
++ |
||
|
2.0 |
10 |
4 |
40±0.56 |
++ |
||
|
2.5 |
10 |
5 |
50±0.63 |
++ |
||
|
3.0 |
10 |
3 |
30±0.72 |
+ |
||
|
3.5 |
10 |
2 |
20±0.84 |
+ |
||
|
4.0 |
10 |
2 |
20±0.61 |
+ |
Note: + Low callus, ++Moderate callus, +++ High callus
Table 1: Evaluation of different leaf explants of S.acmella for callus induction on MS with different concentrations of 2,4-D
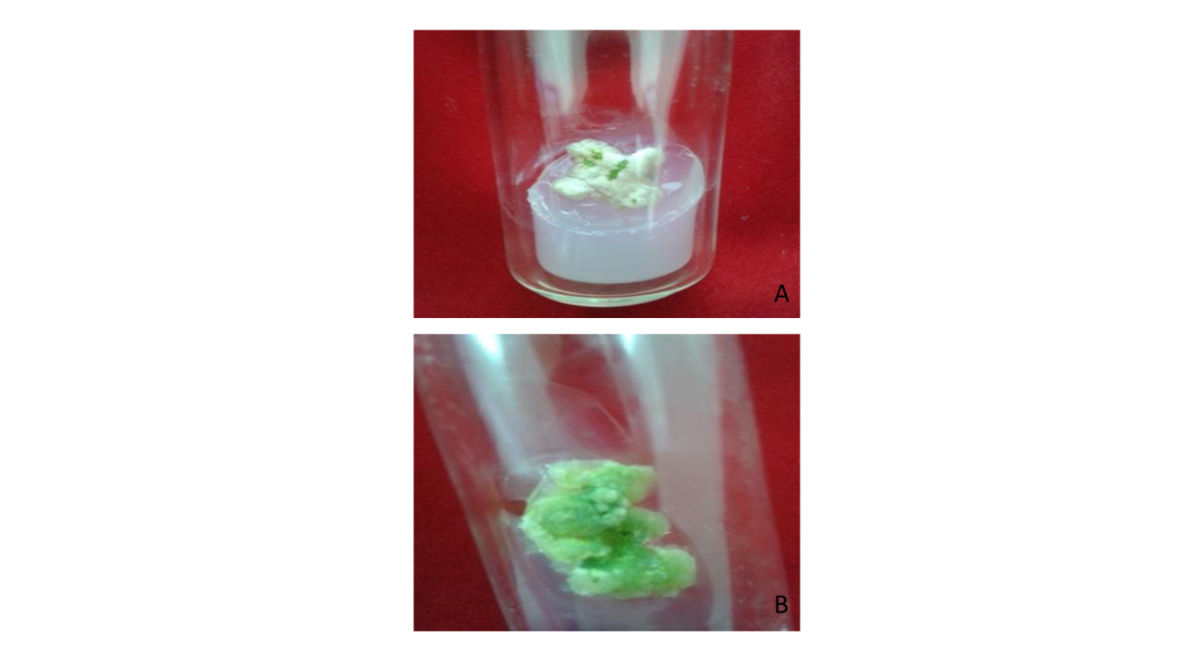
Note: A-Young leaves; B-Mature leaves; C-Old Leaves
Plate 1: Induction of Callus from different Leaf explants of S.acmella on MS with 2.5 mg/l 2,4-D
Callus Induction from Nodal Explants of S.acmella
Callus was also induced from young, mature and old nodal segments of S.acmella on different concentrations of 2,4-D alone with varying frequencies. In young nodal explants, callus was induced within 10 days of inoculation. The mature and old leaves showed callus induction within 14 and 16 days of inoculation respectively.
For the young nodal segments, the percentage of callus induction ranged from 20 to 90 % in different concentrations of 2.4-D. At lower concentration of 2,4-D ( 0.5 to 2.0 mg/l ), the frequency of callus varied from 70 to 90 %. Further increase in the concentration of 2, 4-D (2.5 to 4.0 mg/l), the percentage of callusing decreased (80 to 20%). The quality of the callus was moderate at lower concentration of 2, 4-D. High quality callus was observed at concentrations 1.5 mg/l to 2.5 mg/l 2,4-D. At 4.0 mg/l 2, 4-D, the quality and percentage of callus was low (20%). Among all combinations, high frequency of good callus (90%) was observed at 2 mg/l 2, 4-D.
For the mature nodal segments, the frequency of callus induction ranged from 20 to 80% in different concentrations of 2, 4-D. The percentages of callusing was higher (60 to 80%) at low concentrations of 2, 4- D (0.5 to 2.0 mg/l). The moderate quality of callus was noticed at lower and higher concentrations of 2, 4-D. However, less percentage of callus with low quality was observed at 4.0 mg/l 2, 4-D. High percentage of callus (70 and 80%) with good quality was induced at 1.5 mg/l and 2.0 mg/l 2,4-D respectively.
For the old nodal segments, low percentage (10 to 40%) moderate quality of callus was observed at different concentrations of 2, 4-D. The percentage of callus is 30 % at different concentrations of 2,4-D i.e. 0.5 to 3.5 mg/l. Less callusing and low quality callus was noticed at the concentrations 2.5 mg/l to 3.5 mg/l 2,4-D. However, the callus was negligible (10%) with low quality at 4.0 mg/l 2,4-D.
Among all, 90 % callusing was observed in young nodal explants, 80% for mature and 40% for old nodal segments. Of different concentrations, 2,4-D at 2.0 mg/l gave the highest percentage of callus induction in all three types of explants.
Standardization of Media for Callus Induction
To standardize the media for callus induction, and its growth, the young leaf explants were selected as they gave good callusing and highest percentage of callusing. The young leaf explants were inoculated onto various media with 2.0 mg/l 2,4-D (Table 2).
|
Media |
Explants inoculated |
Explants responded |
Callus induction (%) |
Observation |
|
MS media |
20 |
18 |
90.0±0.28 |
Green, Rapidly proliferating, Granular callus |
|
SH media |
20 |
16 |
80.0±0.25 |
White, Proliferating, Friable callus |
|
Gamborg’s media |
20 |
15 |
75.0±0.46 |
White, Proliferating, Compact callus |
|
MS media with B5 vitamins |
20 |
13 |
72.0±0.45 |
White, Proliferating, Granular callus |
Table 2: Standardization of media for callus induction from leaf explants of S.acmella with 2mg/l 2, 4-D
The leaf segments enlarged in their size followed by callusing from the cut ends within 10 days of inoculation. Among the four different media i.e. MS, SH, Gamborg’s media and MS media with B5 vitamins used, MS media showed good response in terms of frequency of callusing (90%). The callus was green, rapidly proliferating and granular. The SH media resulted in 80% callusing with white, proliferating and friable callus. In Gamborg’s media comparatively less callusing was observed (75%) which is white, proliferative and compact. The MS with B5 vitamins resulted in white, proliferative and granular callus (72%). Among all, MS media showed high frequency of callus (90%) and further experiments were carried with this media only.
|
S.No. |
Explants |
Hormone 2,4-D mg/l |
No. of explants inoculated |
No. of explants responded |
Callus induction % |
Quality of Callus |
|
1. |
Young Nodal segments |
0.5 |
10 |
7 |
70±0.22 |
++ |
|
1.0 |
10 |
7 |
70±0.56 |
++ |
||
|
1.5 |
10 |
8 |
80±0.72 |
+++ |
||
|
2.0 |
10 |
9 |
90±0.68 |
+++ |
||
|
2.5 |
10 |
8 |
80±0.25 |
++ |
||
|
3.0 |
10 |
6 |
60±0.34 |
++ |
||
|
3.5 |
10 |
5 |
50±0.44 |
++ |
||
|
4.0 |
10 |
2 |
20±0.22 |
+ |
||
|
2. |
Mature Nodal segments |
0.5 |
10 |
6 |
60±0.34 |
++ |
|
1.0 |
10 |
6 |
60±0.55 |
++ |
||
|
1.5 |
10 |
7 |
70±0.67 |
+++ |
||
|
2.0 |
10 |
8 |
80±0.45 |
+++ |
||
|
2.5 |
10 |
7 |
70±0.58 |
++ |
||
|
3.0 |
10 |
6 |
60±0.54 |
++ |
||
|
3.5 |
10 |
5 |
50±0.34 |
++ |
||
|
4.0 |
10 |
2 |
20±0.23 |
+ |
||
|
3. |
Old Nodal segments |
0.5 |
10 |
3 |
30±0.42 |
++ |
|
1.0 |
10 |
3 |
30±0.28 |
++ |
||
|
1.5 |
10 |
3 |
30±0.56 |
++ |
||
|
2.0 |
10 |
5 |
30±0.72 |
++ |
||
|
2.5 |
10 |
5 |
40±0.55 |
+ |
||
|
3.0 |
10 |
5 |
30±0.43 |
+ |
||
|
3.5 |
10 |
4 |
20±0.22 |
+ |
||
|
4.0 |
10 |
1 |
10±0.30 |
+ |
Note: + Low Callus, ++moderate callus, +++ high callus
Table 3: Evaluation of different nodal explants of S.acmella for callus induction on MS with different concentrations of 2, 4-D
Note: A - Young nodal segments; B –Mature nodal segments; C - Old nodal segments
Plate 2: Induction of Callus from different Nodal explants of S.acmella on MS with 2.5 mg/l 2,4-D
Effect of Different Growth Regulators on Callusing from Leaf Explants
Young leaves produced good amount of callus on MS media supplemented with various concentrations of 2,4-D. However, to further improve the quality and frequency of callusing, different concentrations of 2,4-D (0.5 - 3.0 mg/l) in combination with BAP (0.5 mg/l) was tested. Also, various concentrations of NAA (0.5 to 3.0 mg/l) in combination with BAP (0.5 mg/l) were evaluated for induction of callusing (Table 4 and Plate 4).
In different combinations of 2,4-D with BAP, the percentage of callusing ranged from 60 to 95%. Among all, 2 mg/l 2,4-D with 0.5 mg/l BAP produced the highest percentage of callus (95%) which is greenish yellow and granular. The combination 0.5 mg/l 2,4-D + 0.5 mg/l BAP produced greenish yellow fragile callus with 60% callus induction. The concentration of 1.0 mg/l 2,4-D + 0.5 mg/l BAP produced greenish yellow granular callus with 70% callus induction. At 3.0 mg/l 2,4-D + 0.5 mg/l BAP, greenish yellow fragile callus resulted with 80% callus induction.
The different combinations of NAA and BAP induced callus with varying frequencies (70 to 87%). Among all, 3 mg/l NAA along with 0.5 mg/l BAP induced high frequency of callus (87%), which is dark green and compact. The concentration 0.5 mg/l NAA+ 0.5 mg/l BAP produced 70% callus which is yellowish green fragile. The concentration 1.0 mg/l NAA+0.5 mg/l BAP produced 80% of yellowish green compact callus. The 2.0 mg/l NAA + 0.5 mg/l BAP produced 84% light green compact callus.
|
S.No. |
Hormonal conc. (mg/l) |
Callus Induction (%) Mean±SE |
Quality of Callus |
Type of Response of callus |
|
1 |
0.5 2,4-D+ 0.5 BAP |
60±0.23 |
++ |
Greenish yellow, fragile |
|
2 |
1.0 2,4-D+ 0.5 BAP |
70±0.67 |
++ |
Greenish yellow, granular |
|
3 |
2.0 2,4-D+ 0.5 BAP |
95±0.84 |
++ |
Greenish yellow, Granular |
|
4 |
3.0 2,4-D + 0.5BAP |
80±0.76 |
++ |
Greenish yellow, Fragile |
|
5 |
0.5 NAA + 0.5 BAP |
70±0.65 |
++ |
Yellowish green, Fragile |
|
6 |
1.0 NAA + 0.5 BAP |
80±0.44 |
++ |
Yellowish green, Compact |
|
7 |
2.0 NAA + 0.5 BAP |
84±0.56 |
++ |
Light green, Compact |
|
8 |
3.0NAA + 0.5 BAP |
87±0.72 |
+++ |
Dark green, Compact |
Note: ++moderate callus, +++ high callus
Table 4: Effect of different growth regulators on callus induction from young leaf explants of S.acmella
Note: A - On MS with 2.0 mg/l 2,4-D + 0.5 mg/l BAP; B - On MS with 3.0 mg/l NAA + 0.5 mg/l BAP
Plate 3: Effect of different growth regulators on callus induction from young leaf explants of S.acmella
DISCUSSION
In this study among all, MS media showed high frequency of callusing (90%), followed by SH (80%) and Gamborg’s media (75%). In MS with B5 vitamins, comparatively less callusing was observed (75%). Several workers also observed that MS was more suitable for callusing over the other media [6]. The superiority of MS medium for callusing over other media may be due to its lower nitrogen content.
In the present work, induction of callus with varying frequencies was observed from young, mature and old leaves on different concentrations of 2,4-D. Many of the previous reports have proved that 2,4-D was the suitable choice of auxins for callus induction. In a study, 2,4-D at 3.0 mg/l gave 80% of callus induction from the leaf explants of S.acmella. Similarly, Rao reported that MS medium with 2.0 mg/l 2,4-D was the optimum for induction of callus from leaf segments in Physalis pubescens [7]. In a previous report on Glinus lotoidis, the best responses for callus induction was observed on the medium containing 0.5 to 3.5 mg/l 2,4-D and at the highest concentration of 2,4-D (4 mg/l), callus induction was reduced [8].
The 2,4-D at 2.0 mg/l was found to give the highest percentage of callus induction in young nodal segments (90%) followed by mature nodal (80%) and old nodal segment(40%). Earlier studies on S.acmella have reported highest percentage of callus induction (80%) from nodal explant with 3.0 mg/l of 2,4-D but the comparative study of callus inductioxn from young, mature and old nodal explants was not reported earlier [9]. Among the two explants, high callusing frequency was observed from young leaf explants than nodal explants. Young explants exhibited better response as these are physiologically and biochemically more active as well as they have less rigid cell wall [10]. Hence, callus from young leaves were used in all other experiments.
To further improve the quality and frequency of callusing from young leaves, different concentrations of 2,4-D (0.5-3.0 mg/l) in combination with BAP (0.5 mg/l) and NAA (0.5-3.0 mg/l) with BAP (0.5 mg/l) were also tested. To achieve maximum callus induction, a defined auxin cytokinin ratio was required, as advocated by Junaid in Catharanthus roseus [11]. The BAP at 0.5 mg/l in combination with different concentrations of 2,4-D was tested for callus induction from leaf explants. Among all, 0.5 mg/l BAP in combination with 2.0 mg/l 2,4-D produced high percentage of greenish yellow callus (95%) , followed by 80% at 3.0 mg/l 2,4-D and 70% at 1.0 mg/l 2,4-D. Similar observation has been reported in Glinus lotoidis L., in which 96.6% of callus formation was observed on the medium containing 2,4-D (1.0 to 4.0 mg/l) combined with 0.5 mg/l BAP [8].
The combination of various concentrations of NAA and BAP was tested for the production of callus from the leaf explants. Among all, 3 mg/l NAA + 0.5 mg/l BAP produced high frequency of dark green and compact callus (87%) which was followed by 84% light green compact callus at 2.0 mg/l NAA + 0.5 mg/l BAP and 80% yellowish green compact callus at 1.0 mg/L NAA+0.5 mg/L BAP. In an earlier report, the better response for callus formation (89%) was observed for S.acmella in the MS supplemented medium with BAP and NAA (0.5mg/l + 3.0 mg/l), but the callus observed was white and friable [12].
CONCLUSION
Culture media are largely responsible for the in vitro growth and morphogenesis of plant tissues. Each explant requires different chemical constituents and the success of the plant tissue culture depends on the choice of the nutrient medium.
The nature and structure of the callus determines the morphogenic potential of the explants. The protocol for callus induction has been standardized using different plant parts like nodal and leaf explants. Among different explants tested, young leaves and nodal segments exhibited better response in terms of quality and frequency of callus indicating that successful callus induction depends upon the physiological state and inherent capacity of cells in the callus. The callus culture protocols established from S.acmella can be used for the production of secondary metabolites. This protocol can also be further explored to produce the in vitro plantlets of S.acmella.
REFERENCES
- Nurul Izzati Osman NI, Asmah Awal AA, Norrizah Jaafar Sidik NJ, Shamsiah Abdullah SA . Callus induction and somatic embryogenesis from leaf and nodal explants of Lycium barbarum(Goji). [Crossref] [Google Scholar]
- Dubey S, Maity S, Singh M, Saraf SA, Saha S. Phytochemistry, pharmacology and toxicology of Spilanthes acmella: a review. Adv Pharmacol Sci. 2013(1):423750. [Crossref] [Google Scholar] [PubMed]
- Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V. High therapeutic potential of Spilanthes acmella: a review. EXCLI journal. 2013;12:291. [Google Scholar] [PubMed]
- Rao NK, Reddy RK. Threatened plants of Tirupati and its environs. An Assessment of Threatened Plants of India. Deptartment of Environment, Howrah. 1983:167-8. [Google Scholar]
- Shiwali Sharma SS, Anwar Shahzad AS. Efficient micropropagation of Spilanthes acmella (L.) Murr.: a threatened medicinal herb. [Crossref] [Google Scholar]
- Zheng S, Henken B, Sofiari E, Jacobsen E, Krens FA, et al. Factors influencing induction, propagation and regeneration of mature zygotic embryo-derived callus from Allium cepa. Plant cell, tissue and organ culture. 1998;53(2):99-105. [Crossref] [Google Scholar]
- Rao SR, Ravishankar GA. Plant cell cultures: chemical factories of secondary metabolites. Biotechnology advances. 2002;20(2):101-53. [Crossref] [Google Scholar] [PubMed]
- Shiferaw Teshome ST, Tileye Feyissa TF. In vitro callus induction and shoot regeneration from leaf explants of Glinus lotoides (L.)-an important medicinal plant. [Crossref] [Google Scholar]
- Yadav K, Singh N. Micropropagation of Spilanthes acmella Murr.–An important medicinal plant. Nature and Science. 2010;8(9):5-11. [Google Scholar]
- Misra AK, Bhatnagar SP. Direct shoot regeneration from the leaf explant of cucumber (Cucumis sativus L.). [Google Scholar]
- Junaid A, Mujib A, Bhat MA, Sharma MP, Šamaj J. Somatic embryogenesis and plant regeneration in Catharanthus roseus. Biologia plantarum. 2007;51(4):641-6. [Crossref] [Google Scholar]
- Chaitali Niratker CN, Malti Singh MS, Preeti P. Callus induction and plant regeneration from leaf explants of Spilanthes acmella Murr.: an endangered medicinal plant. [Google Scholar]
Article Processing Timeline
| 2-5 Days | Initial Quality & Plagiarism Check |
| 15 Days |
Peer Review Feedback |
| 85% | Acceptance Rate (after peer review) |
| 30-45 Days | Total article processing time |
Indexed In
ResearchBib
Sindexs
OAJI
DOAJ
CrossRef
PubMed
MEDLINE
EBSCO A-Z / Host
OCLC - WorldCat
Journal Flyer